What is 2,4-Dinitrophenylhydrazine (2,4 DNP Test)?
Brady’s reagent (2,4-dinitrophenylhydrazine) is a red-orange solid, usually supplied wet to reduce the risk of explosion.
Table of Contents
- 2,4-Dinitrophenylhydrazine Structure
- Synthesis of 2,4-Dinitrophenylhydrazine
- Brady’s Reagent
- Identifying a Carbonyl Compound
- 2,4-Dinitrophenylhydrazine Laboratory Test
- Reaction with Ethanal
- Frequently Asked Questions – FAQs
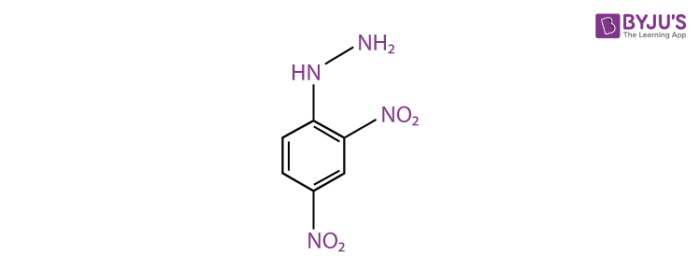
2,4-Dinitrophenylhydrazine Structure
The structure of 2,4-Dinitrophenylhydrazine is given below.
2,4-DNP derivatives offer a convenient way of separating the components of a mixture of aldehydes and ketones. Rather than attempting to do this directly by fractional distillation, a chemist might take advantage first of the easy separation of 2,4-DNP derivatives by column chromatography. Since their formation from aldehydes and ketones is reversible, hydrolysis of the separated derivatives will then regenerate the original carbonyl compounds.
Synthesis of 2,4-Dinitrophenylhydrazine
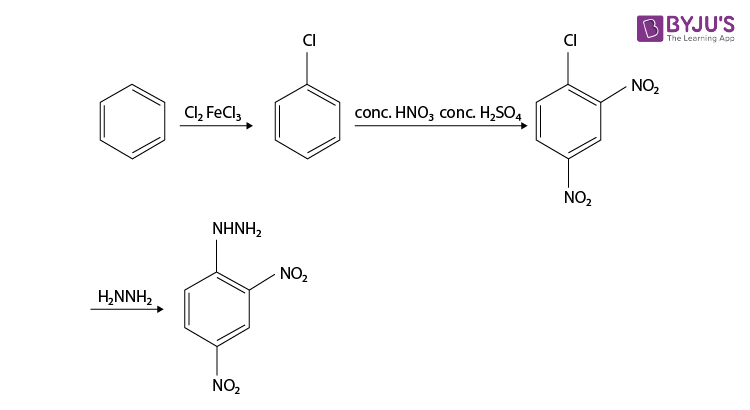
Although it’s not a familiarly substituted hydrazine, we can make a reasonable inference that hydrazine H2N-NH2 is a good nucleophile. So the synthesis of 2,4-Dinitrophenylhydrazine can be done from the product, it is prepared by reacting hydrazine with 2,4-dinitrochlorobenzene. The electron-accepting effect of the two nitro groups makes this chloride easy to displace.
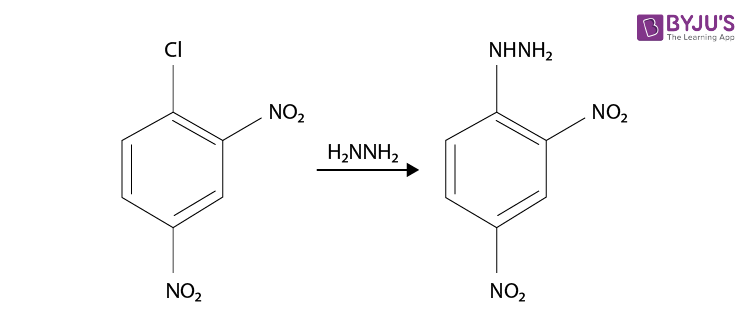
Since chlorine is ortho, para directing and deactivating, so we should nitrate chlorobenzene. Chlorobenzene is readily prepared from benzene itself, so the synthesis is complete and the reaction is given below.
Brady’s Reagent
An aqueous solution of 2,4-dinitrophenyl hydrazine (DNP) is known as Brady’s reagent. It reacts with carbonyl compounds (aldehydes and ketone) to give a coloured precipitate. These precipitates have a sharp melting point. The melting points of the precipitates confirm the carbonyl compounds.
Benzoic acid is used as an antiseptic in medicines meant for urinary disorders and in vapour form for disinfecting bronchial tubes.
- Acetic anhydride reacts with N2O5 to give acetyl nitrate.
(CH3CO)2O + N2O5 → 2CH3COONO2 (Acetyl nitrate)
- The reaction of the acid chloride with water decreases with the increase of C-atoms in alkyl groups.
CH3COCl > CH3-CH2-COCl > CH3-CH2-CH2COCl > ………
Identifying a Carbonyl Compound
The carbonyl compound is simply mixed with an acid solution of Brady’s reagent in methanol. The derivatives are orange coloured crystalline solids called 2,4-dinitrophenylhydrazones. These crystals are filtered off and purified by re-crystallisation.
Their melting temperatures are measured. The original carbonyl compound is then identified by comparing to the tables of melting points of 2,4-dinitrophenylhydrazine derivatives.
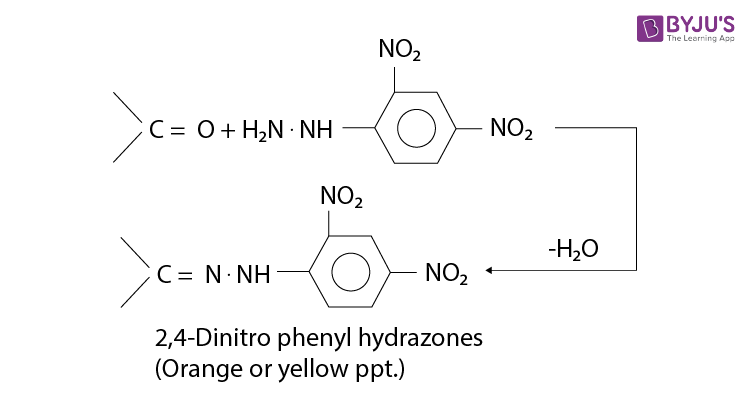
Brady’s reagent can also be used as a test for the presence of a carbonyl compound because orange crystals appear when it is added to either an aldehyde or a ketone.
2,4-Dinitrophenylhydrazine Laboratory Test
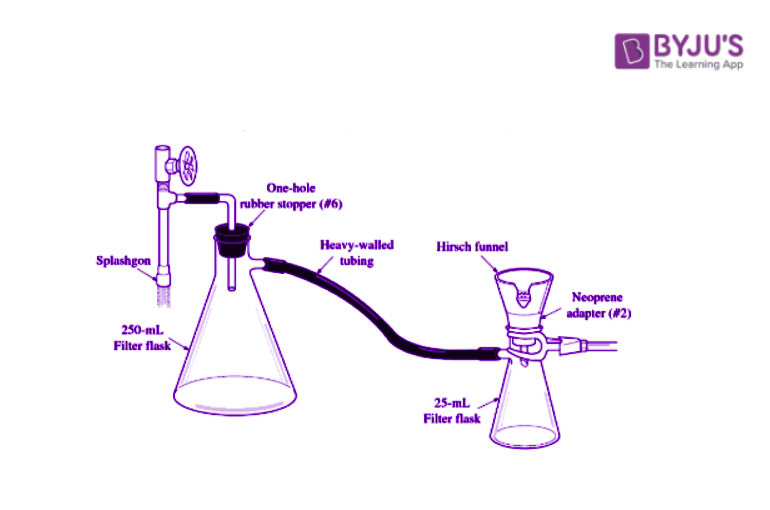
- Place 5ml of the 2,4-dinitrophenylhydrazine reagent in a test tube.
- Add 10 drops of the unknown compound; sharply tap the test tube with your finger to mix. If crystals do not form immediately, gently heat in a water bath (60oC) for 5min.
- Cool in an ice bath until crystals form. Collect the crystals by vacuum filtration using Hirsch funnel.
- Allow the crystals to dry on the Hirsch funnel by drawing air through the crystals. Take a melting point and record on the Report sheet.
- The crystals are usually pure enough to give a good melting point. However, if the melting point range is too large, recrystallize from a minimum volume of ethanol.
2,4-Dinitrophenylhydrazine Reaction with Ethanal
2,4-Dinitrophenylhydrazine reacts with both aldehydes and ketones to form a 2,4-dinitrophenylhydrazone. For example with ethanal;
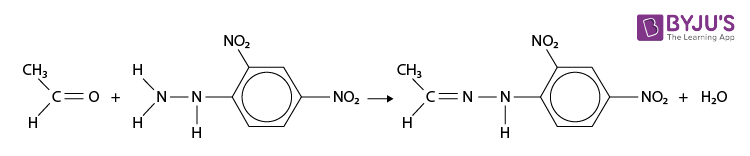
- The reaction is a condensation reaction (water is eliminated). The product is named using the name of the aldehyde or ketone followed by 2,4-dinitrophenylhydrazone, for example, ethanal 2,4-dinitrophenylhydrazone.
- 2,4-dinitrophenylhydrazine can be used as a method for identifying aldehydes and ketones – the 2,4-dinitrophenylhydrazone formed is a solid that can be purified and its melting point determined. Comparison of the melting point with a table of known values could identify the aldehyde or ketone.
- The 2,4-dinitrophenylhydrazone derivatives are all orange/yellow solids.
- The solution of 2,4-dinitrophenylhydrazine is often called Brady’s reagent.
Frequently Asked Questions – FAQs
What does a positive 2,4 DNP test indicate?
2,4-Dinitrophenylhydrazine can be used for the qualitative identification of ketone or aldehyde functional group carbonyl functionality. A positive test is indicated by the formation of a precipitate known as dinitrophenylhydrazone, yellow, orange, or red.
How can you tell the difference between aldehydes and ketones?
The distinction between the aldehyde and ketone is the presence in the aldehyde of a hydrogen atom bound to the double carbon-oxygen bond. Ketones aren’t hydrogen. The presence of the hydrogen atom makes it very simple to oxidize aldehydes, they are fast reduction agents.
Why are 2,4 Dinitrophenylhydrazones better derivatives than Phenylhydrazones?
2,4-Dinitrophenylhydrazones are, for different reasons, safer derivatives than phenylhydrazones. First, there are higher molecular masses in these derivatives, increasing the volume of substance to be checked. Heavier-mass derivatives also have a greater chance of becoming a solid.
What will a DNP test determine and how?
2,4-Dinitrophenylhydrazine can be used for the qualitative identification of ketone or aldehyde functional group carbonyl functionality. A successful test is indicated by the formation of a precipitate yellow, orange, or red known as dinitrophenylhydrazone.
What is Brady’s reagent?
The chemical compound C6H3(NO2)2NHNH2 is 2,4-Dinitrophenylhydrazine (DNPH, Brady’s reactive, Borche’s reagent). Dinitrophenylhydrazine is a solid whose colours can range from red to white. It is a synthetic hydrazine-derivative, which is mostly used for the qualitative research of aldehyde which is a ketone related carbonyl group.
Does alcohol react with Brady’s reagent?
Aldehydes and ketones react to yellow, orange, or reddish-orange precipitates with the 2,4-dinitrophenylhydrazine reagent, whereas alcohols do not react. This is a successful technique for checking for the existence of a drug or demonstrating its absence.
Why does glucose not give a 2,4 DNP test?
That’s because glucose doesn’t react with Schiff’s reagent & 2,4DNP reagent despite having an aldehydrated group. It forms either α-anomer after the internal cycling. There is no free aldehyde group present in these forms. Thus, it does not give an aldehyde group reaction.