What is Acetamide?
C2H5NO is an organic compound with chemical name Acetamide.
Acetamide is also called Acetic acid amide, or Ethanamide or Acetimidic acid. It is derived from acetic acid and is the simplest amide. It is widely used as a plasticizer.
Ethanamide is obtained as a hygroscopic solid which is colourless and has a mousy odour. It is readily soluble in water, chloroform, hot benzene, glycerol and slightly soluble in ether. It is a member of the class of acetamides which results from the formal condensation of acetic acid (CH3COOH) with ammonia (NH3). It is naturally found in red beetroot.
Table of Content
Structure of Acetamide -CH3CONH2
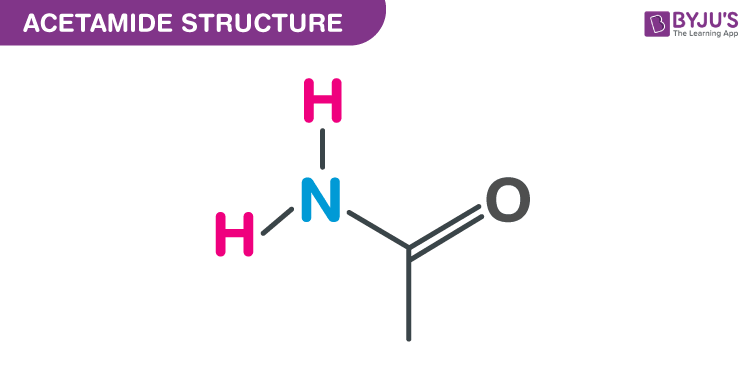
Acetamide Structure – C2H5NO
Properties of Acetamide –CH3CONH2
Production of Acetamide-CH3CONH2
In chemical laboratories, it can be produced by dehydration of ammonium acetate. The reaction is as follows:
[NH4][CH3CO2] → CH3C(O)NH2 + H2OIt can also be obtained through ammonolysis of acetylacetone with the under conditions that are used in reductive amination.
Alternately, it can be produced from anhydrous acetic acid (CH3COOH), dried hydrogen chloride gas, and acetonitrile in an ice bath along with a reagent acetyl chloride.
On an industrial scale, it can be produced by dehydrating ammonium acetate or by hydrolyzing acetonitrile.
CH3CN + H2O → CH3C(O)NH2
Uses of Acetamide-CH3CONH2
- Acetamide is used as a solvent for many inorganic and organic compounds.
- Used in explosives.
- Used as a plasticizer.
- Used as a hygroscopic agent.
- Used to manufacture methylamine.
- Used as a stabilizer.
- Used as a penetrating agent.
- Used as a fire suppressant.
Health Hazards of Acetamide- CH3CONH2
It is combustible and generates toxic gas or fumes when heated. Exposure to Acetic acid amide may cause irritation to the mucous membranes, skin and eyes. It can also cause corneal damage.
Recommended Videos
Carboxylic Acids AcidityFrequently Asked Questions
What is acetamide used for?
Acetamide is an organic compound that has the CH3CONH2 formula. This is the simplest amide of acetic acid derivatives. Acetamide is used in the manufacture of polymeric products, such as polyvinyl acetamide, a polymeric commodity used as an absorbent, as a co-monomer.
Why is acetamide soluble in water?
The primary amide is formed from NH2, amino group replacing the carboxylic hydroxyl group. A case in point is acetamide (acetic acid + amide). Low molecular weight amides caused by the formation of hydrogen bonds are soluble in water.
What does acetamide smell like?
Acetamide is an acetic acid-derived chemical that has been identified as smelling like vinegar or ammonia. It will cling to places where the musculus is sleeping and going to get food scrounge.
Is acetamide soluble in HCl?
The key findings have been that ammonia and acetamide are water and HCl soluble since they are smaller molecules. The compounds triethylamine, aniline, and N, N-dimethylaniline are not known to be water- and HCl soluble, but are MTBE-soluble. MTBE is not soluble in ammonia and acetamide.
Why is acetamide a weaker base than ethylamine?
The presence of a lone pair of electrons on a base determines its intensity as these electrons are the ones that will “mop up” H+ ions in solution and thus increase pH toward more alkaline conditions. Phenylamine is also a weaker base than ethylamine since there is less of a lone pair.